Introduction:The Monkeypox Virus Detection Kit (Fluorescence PCR) is a qualitative in vitro real-time PCR test for the detection of nucleic acid from monkeypox virus in oropharyngeal swab, nasopharyngeal swab, serum, plasma and human pustular or vesicular rash specimens from individuals suspected of monkeypox virus infectious. Results are for the identification of monkeypox virus DNA. Positive results are indicative of the presence of monkeypox virus DNA; clinical correlation with patient history and other diagnostic information is necessary to determine patient infection status. Positive results do not rule our bacterial infection or co-infection with other virus. The agent detected may not be the definite cause of disease. Negative results do not preclude monkeypox virus infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical obesrvations, patient history, and epidemiological information. The Monkeypox Virus Detection Kit (Fluorescence PCR) is intended for use by qualified and trained clinical laboratory personnel specifically instructed and trained in the techniques of real-time PCR and in vitro diagnostic procedures.
Components of the Kit: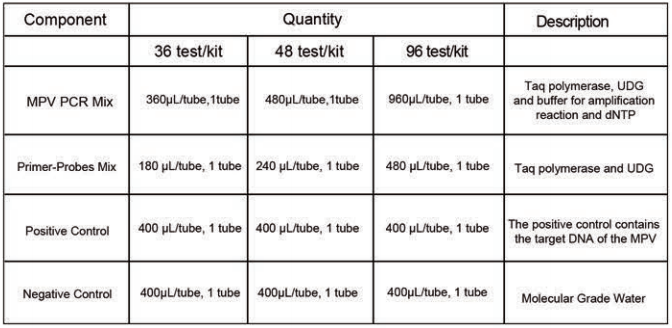 Specification:
Specification:
| Cat.NO. |
Production Name |
Packing Size |
Storage |
Shelf Life |
| LFP-003A |
Monkeypox Virus Detection Kit (Fluorescence PCR) |
36 Tests/box |
lower than -18℃ |
12 months |
| LFP-003B |
48 Tests/box |
| LFP-003C |
96 Tests/box |
